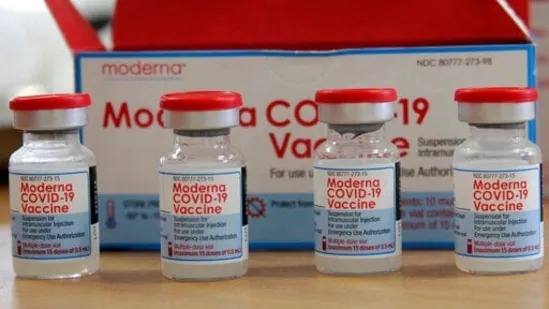
Japan suspended the utilization of 1.63 million doses of Moderna Inc’s Covid-19 vaccine on Thursday, quite every week after the domestic distributor received reports of contaminants in some vials.
Both Japan and Moderna said that no safety or efficacy issues had been identified which the suspension was just a precaution. But the move prompted several Japanese companies to cancel worker vaccinations planned for Thursday.
“Moderna confirms having been notified of cases of material being seen in drug product vials of its Covid-19 vaccine,” Moderna said during a statement.
“The company is investigating the reports and remains committed to working expeditiously with its partner, Takeda, and regulators to deal with this,” it added, pertaining to Japan’s Takeda Pharmaceutical, which distributes the vaccine within the country.
A Health ministry official said Takeda first discovered about the contaminated vials on Aug. 16 and reported the problem to the govt. on Wednesday. The delay was because Takeda needed time to assemble information on which vials were affected and where they were within the country, the official said.
Moderna said the contamination might be thanks to a producing issue in one amongst the assembly lines at its contract manufacturing site in Spain.
Spanish pharma company Rovi, which bottles or “fills and finishes” Moderna vaccines for markets aside from the us, said it’s investigating possible contamination of Moderna doses and therefore the issue seemed to be limited to some batches bound for Japan