 Expanded polystyrene foam (EPF) is a plastic material that has special properties due to its structure. Composed of individual cells of low density polystyrene, EPF is extraordinarily light and can support many times its own weight in water. Because its cells are not interconnected, heat cannot travel through EPF easily, so it is a great insulator. EPF is used in flotation devices, insulation, egg cartons, flats for meat and produce, sandwich and hamburger boxes, coffee cups, plates, peanut packaging, and picnic coolers. Although it is generally called Styrofoam, Styrofoam is a trademark of Dow Chemical Company and refers specifically to a type of hard, blue EPF used mainly in boating.
Expanded polystyrene foam (EPF) is a plastic material that has special properties due to its structure. Composed of individual cells of low density polystyrene, EPF is extraordinarily light and can support many times its own weight in water. Because its cells are not interconnected, heat cannot travel through EPF easily, so it is a great insulator. EPF is used in flotation devices, insulation, egg cartons, flats for meat and produce, sandwich and hamburger boxes, coffee cups, plates, peanut packaging, and picnic coolers. Although it is generally called Styrofoam, Styrofoam is a trademark of Dow Chemical Company and refers specifically to a type of hard, blue EPF used mainly in boating.
Raw Materials
EPF’s main component is styrene (C 8 H 8 ), which is derived from petroleum or natural gas and formed by a reaction between ethylene (C 2 H 4 ) and benzene (C 6 H 6 ); benzene is produced from coal or synthesized from petroleum. Styrene is polymerized either by heat or by an initiator such as benzoyl peroxide. Stopping the polymerization is difficult; however, inhibitors such as oxygen, sulfur, or quinol can be used. To form the low-density, loosely attached cells EPF is noted for, polystyrene must first be suspended in water to form droplets. A suspension agent, such as specially precipitated barium sulfate or copolymers of acrylic and methacrylic acid and their esters (organic product formed by the reaction between of an acid and an alcohol), is then added to the water. Numerous suspension agents are used commercially. All are similarly viscous and serve to hold up the droplets, preventing them from sticking together. The beads of polystyrene produced by suspension polymerization are tiny and hard. To make them expand, special blowing agents are used, including propane, pentane, methylene chloride, and the chlorofluorocarbons.
Design
Like all plastics, EPF consists of a polymer chain with great molecular weight. A molecule‘s weight is equivalent to its mass and can be calculated by adding the mass of its constituent atoms. EPF is a linear polymer whose basic unit is styrene (C 8 H 8 ) and whose molecular mass is 104, yet when it is linked together as it is in the plastic, its mass can range between 200,000 and 300,000 (because a polymer chain can contain an indefinite number of molecular links, a terminal mass cannot be determined).
The Manufacturing
Process
First, styrene is made by combining ethylene and benzene. Next, the styrene is subjected to suspension polymerization and treated with a polymerization initiator, which together convert it into polystyrene. Once a polymer chain of the desired length has formed, technicians stop the reaction with terminating agents. The resulting polystyrene beads are then cleaned, and anomalous beads filtered out. To make small-cell EPF, workers then melt, add a blowing agent to, and extrude the beads. To produce smooth-skinned EPF, they pre-expand the beads, dramatically reducing their density. Next they heat and expand them before allowing them to sit for 24 hours so that they can cool and harden. The beads are then fed into a mold of the desired shape.
Making styrene
- 1 The basic unit of polystyrene is styrene, which is the product of a two-fold reaction. Ethylene and benzene, in the presence of a catalyst such as aluminum chloride, form ethylbenzene (C 8 H 8 ), which is then dehydrogenated (hydrogen is removed) at 1,112-1,202 degrees Fahrenheit (600-650 degrees Celsius) to form styrene (C 8 H 8 ).
Making polystyrene
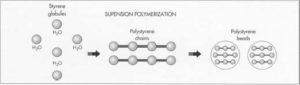
- 2 Polystyrene is formed from styrene through suspension polymerization, a process by which tiny drops of the monomer (in this case, styrene) are completely surrounded by water and a mucilaginous substance. Supporting and surrounding the styrene globules, the suspension agent produces uniform droplets of polystyrene.
- 3 Next, a polymerization initiator is added to the droplets, which are suspended by heat radiation of about 212 degrees Fahrenheit (100 degrees Celsius). This results in free radicals, a group of atoms particularly likely to react with others because they contain unpaired electrons which are available for molecular bonding. Free radicals then combine at randomly to form chains of polystyrene.
- 4 Stopping the polymerization process is difficult. Terminators are introduced to the process to end it at the appropriate time. Though variable, chain length must fall within a certain range, because polystyrene with overly long chains won’t melt readily, and polystyrene with short chains will be brittle.
Preparing the beads
- 5 After polymerization is complete, the mixture—consisting of beads made up of polystyrene chains—is cooled. These beads are then washed out and dried. Uniform bead size is achieved by sorting the beads through meshes which filter out over- and undersized beads.









